Current Testing Tools Uncompromised by New COVID-19 Variant of Concern Omicron (B.1.1.529)
29 November 2021
- Evidence indicates that PCR and rapid antigen tests can detect the new Omicron (B.1.1.529) variant of concern
- The identification and tracking of new variants, including Omicron, relies on testing and sequencing capacity, which continue to be under-resourced in low- and middle-income countries
GENEVA - The accuracy of existing molecular (PCR, NAAT) tests appears uncompromised by COVID-19 variant Omicron (B.1.1.529), designated a Variant of Concern by the World Health Organization (WHO) on Friday. Preliminary evidence suggests that this is also true for the accuracy of rapid antigen tests. FIND, the global alliance for diagnostics, has conducted a rapid assessment of available evidence in the context of the Access to COVID 19 Tools (ACT) Accelerator Diagnostics Pillar, which they co-lead alongside the Global Fund, with WHO.
The identification of the Omicron variant has sparked growing concerns over its unusually high number of mutations and rapid spread in South Africa. The variant was first identified in Botswana and South Africa, and appears to be spreading rapidly in South Africa, with new cases also now emerging through importations in Asia and Europe. The new variant has over 30 mutations in the spike protein – double that of the dominant Delta variant – which has raised concerns about vaccine efficacy. Some of these mutations have been linked to enhanced transmissibility and the capacity of variants to evade immune responses. Preliminary evidence also suggests that this variant has a greater capacity to reinfect people compared with other variants. Work is ongoing to properly assess the threat it poses and the likelihood that it may drive a new global wave of COVID-19. WHO published a technical brief on enhanced readiness for Omicron on 28 November 2021.
The ability of diagnostic tests to detect the Omicron variant is critical to tracking its spread and putting in place measures to halt transmission. Analyses so far suggest that the rapid antigen (lateral flow) and gold-standard PCR tests should still detect the variant, but more comprehensive verification of this is ongoing.
The new variant contains a deletion in its S gene, one of the three common target genes of many PCR tests. This can cause what’s known as “S gene dropout”, where the PCR S-gene target is not detected, while the other PCR gene targets are still recognized. Therefore, the overall PCR accuracy for tests with multiple genetic targets is not likely to be impacted. The missing S gene target can even act as a surrogate marker for the new variant in tests which target this gene, as the currently dominant Delta variant is S-gene positive on PCR. The S gene dropout has made possible timely screening for the Omicron variant in some regions of South Africa and Botswana, and gives countries a way to track the variant’s spread without genomic sequencing, which is usually only performed for a subset of PCR-positive samples (where capacity is available).
A major issue is that many countries are still struggling with testing capacity for COVID-19, including genomic sequencing capacity. Across Africa, capacity for testing has been complicated by supply chain issues and high costs for essential test reagents as well as limited funding to procure diagnostics. Testing rates in some countries remain worryingly low, creating blind spots where new variants like Omicron can spread unnoticed. Currently only 22.9% of tests administered worldwide have been used in low- and middle-income countries, despite these countries making up half of the global population.
Identifying and closing these testing gaps is critical, and FIND is monitoring both testing and sequencing capacity for COVID-19. An interactive SARS-CoV-2 test tracker shows the current testing situation in each country, and FIND has systematically mapped and evaluated global sequencing capacity. FIND and the Global Fund have been working to build testing and sequencing capacity in Africa and across low-and middle-income countries through the ACT Accelerator Diagnostics Pillar. The Pillar continues to monitor the impact of new variants on diagnostic testing.
The Global Fund has supported low- and middle-income countries with US$718 million for the procurement of COVID-19 diagnostic tests. The Diagnostics Pillar of ACT-Accelerator is working to ensure equitable access to testing tools, stimulate rapid and effective uptake of testing in countries, and drive the development and expansion of affordable, transformative, digitally integrated tests.
Bill Rodriguez, CEO of FIND, said: “The emergence of new COVID-19 variants is inevitable as time goes on. High rates of testing and sequencing allow us to track community spread, offer clinical care and identify new variants like Omicron. Early detection allows us to strengthen our defences before things get out of control. Making sure all countries have equitable access to COVID-19 testing and sequencing capacity is vital and needs to be a global priority to provide proper care and to keep everyone safe from new variants.”
Peter Sands, Executive Director of the Global Fund, said: “Inequitable access to COVID-19 tools – including diagnostic tests, treatments, vaccines, and personal protective equipment – is severely hindering the pandemic response in low- and middle-income countries. Not only is this morally wrong, it also creates the perfect conditions for new variants like Omicron to emerge and spread. To contain this pandemic as well as future ones, we must redouble our efforts to fight this virus everywhere, leaving no one behind. We must act now.”
About FIND
FIND, the global alliance for diagnostics, seeks to ensure equitable access to reliable diagnosis around the world. We connect countries and communities, funders, decision-makers, healthcare providers and developers to spur diagnostic innovation and make testing an integral part of sustainable, resilient health systems. We are working to save 1 million lives through accessible, quality diagnosis, and save US$1 billion in healthcare costs to patients and health systems. We are co-convener of the Access to COVID-19 Tools (ACT) Accelerator diagnostics pillar, and a WHO Collaborating Centre for Laboratory Strengthening and Diagnostic Technology Evaluation. For more information, please visit www.finddx.org
About the Global Fund
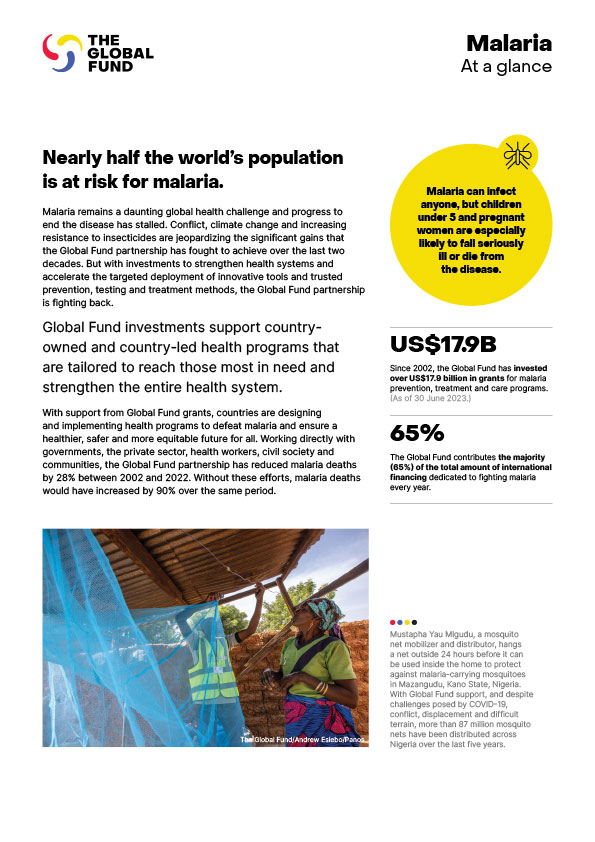
The Global Fund is a worldwide movement to defeat HIV, TB and malaria and ensure a healthier, safer, more equitable future for all. We raise and invest US$4billion a year to fight the deadliest infectious diseases, challenge the injustice which fuels them and strengthen health systems in more than 100 of the hardest hit countries. We unite world leaders, communities, civil society, health workers and the private sector to find solutions that have the most impact, and we take them to scale worldwide. Since 2002, the Global Fund has saved 38 million lives.
About the ACT-Accelerator
The Access to COVID-19 Tools (ACT) Accelerator is a global coalition of organizations developing and deploying the new diagnostics, treatments and vaccines needed to end the acute phase of the pandemic. Pooling the expertise of its many partners, the ACT-Accelerator has quickly ushered in rapid, affordable tests and effective medicines for low and middle-income countries and established the COVAX facility for the equitable procurement and distribution of vaccines in low- and lower-middle-income countries. The ACT Accelerator partnership was formed at the onset of the pandemic in response to a call from G20 leaders, and was launched by WHO, the European Commission, France, and the Bill & Melinda Gates Foundation.