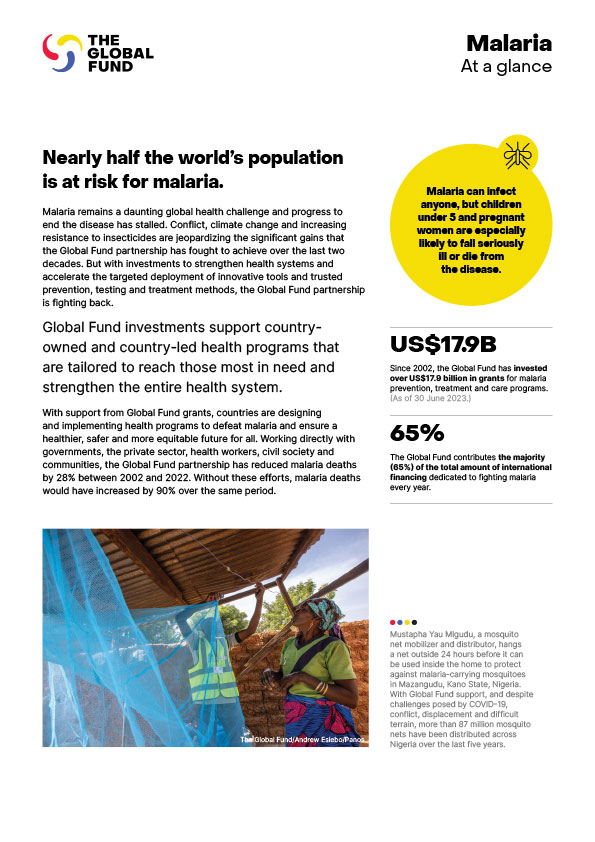
PAXLOVID Now Available Through Global Fund Support
14 October 2022
The new COVID-19 oral antiviral medicine nirmatrelvir/ritonavir, better known as PAXLOVID, is now available to be procured through Global Fund support.
PAXLOVID is strongly recommended by WHO to treat non-severe cases of COVID-19 in patients who are at high risk of hospital admission. PAXLOVID needs to be administered while the disease is at its early stages, meaning that prompt and accurate testing is essential for a successful outcome, as well as good links to care provision for assessment and prescription of the drug.
Implementing countries that are reprogramming C19RM funds can introduce newly recommended therapeutics, or scale-up “test and treat”. The end goal is to accelerate service delivery and scale-up of novel treatments, targeted to the most vulnerable and high-risk populations, in high-burden settings with low vaccination coverage.
Principal Recipients interested in purchasing the new medicine can contact Global Fund Country Teams directly to receive the contractual details of the agreement.
Terms and conditions differ from those currently used for other health products and are applicable between PRs and i+solutions, the Global Fund’s procurement service agent in charge of PPM orders relating to PAXLOVID. PRs will have to accept these terms and conditions in their agreement through a purchase order with i+solutions.